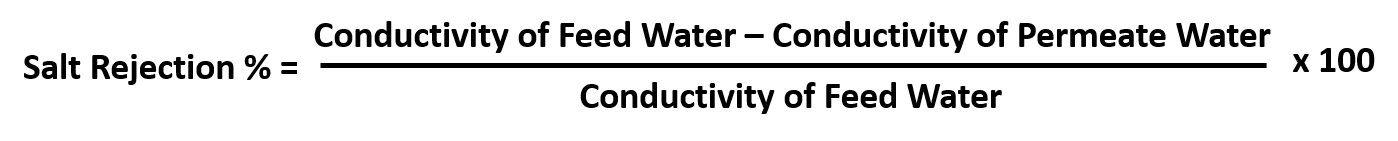
Reverse Osmosis System
Osmosis
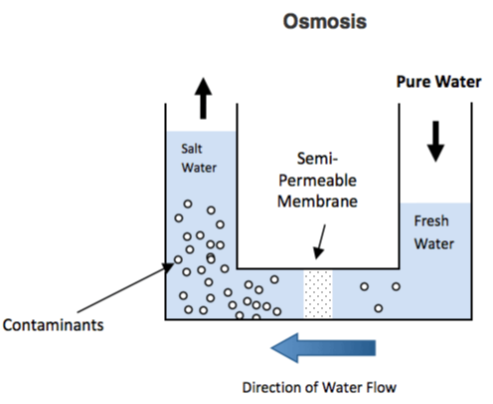
To apprehend the aim and process of reverse osmosis you need to first recognize the evidently happening system of osmosis. Osmosis may be a evidently happening phenomenon and one among the foremost important tactics in nature. It is a procedure in which a weaker saline will tend emigrate to a strong saline answer. Examples of osmosis are while plant roots soak up water from the soil and our kidneys absorb water from our blood.
Below may be a diagram which shows how osmosis works. An answer that's less concentrated may have a herbal tendency emigrate to an answer with a better awareness. As an example, if you had a field complete of water with a coffee salt attention and a few other container filled with water with a excessive salt concentration which they were separated with the help of a semi‐permeable membrane, then the water with the decrease salt attention could start to migrate towards the water box with the higher salt awareness.
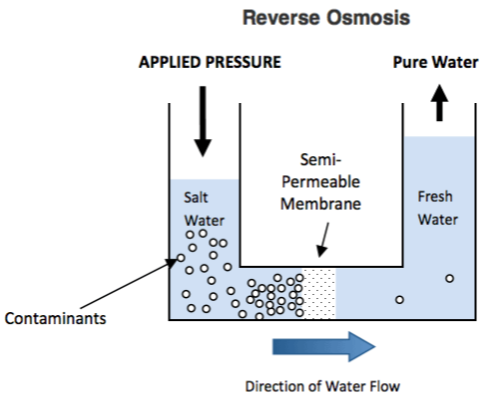
Reverse Osmosis is the process of Osmosis in reverse.
Whereas Osmosis occurs naturally without energy required, to reverse the process of osmosis you need to apply energy to the more saline solution. A reverse osmosis membrane is a semi‐permeable membrane that allows the passage of water molecules but not the majority of dissolved salts, organics, bacteria and pyrogens. However, you need to ‘push’ the water through the reverse osmosis membrane by applying pressure that is greater than the naturally occurring osmotic pressure in order to desalinate (demineralize or deionize) water in the process, allowing pure water through while holding back a majority of contaminants.
Below is a diagram outlining the process of Reverse Osmosis. When pressure is applied to the concentrated solution, the water molecules are forced through the semi‐permeable membrane and the contaminants are not allowed through.
What will Reverse Osmosis remove from water?
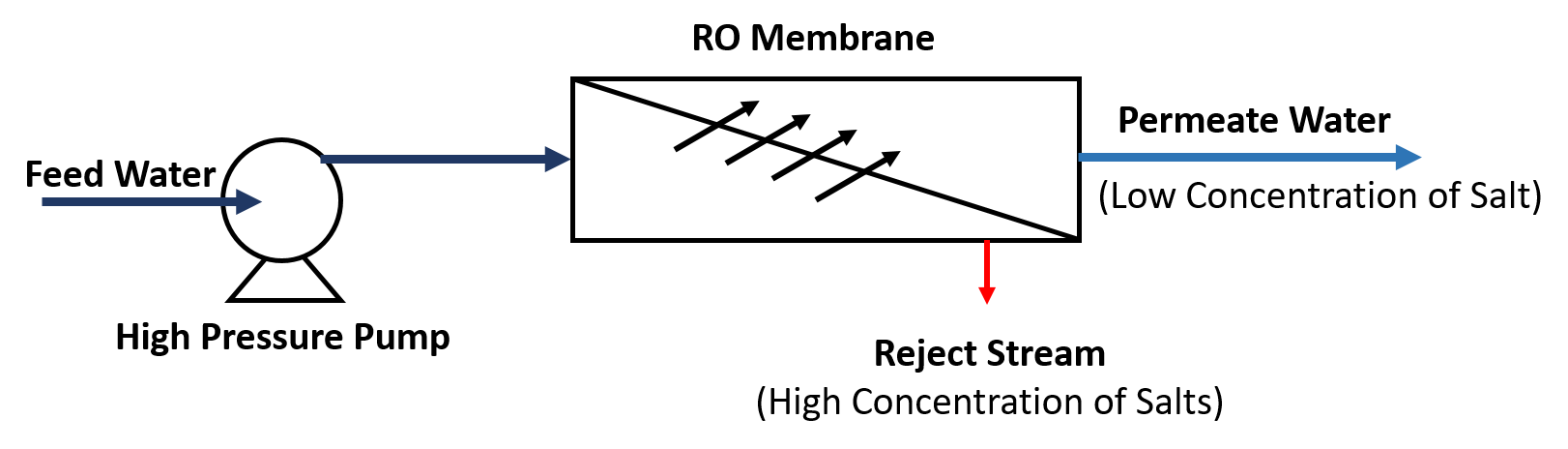
Reverse Osmosis is capable of removing up to 99%+ of the dissolved salts (ions), particles, colloids, organics, bacteria and pyrogens from the feed water (although an RO system should not be relied upon to remove 100% of bacteria and viruses). An RO membrane rejects contaminants based on their size and charge. Any contaminant that has a molecular weight greater than 200 is likely rejected by a properly running RO system.
Likewise, the greater the ionic charge of the contaminant, the more likely it will be unable to pass through the RO membrane. For example, a sodium ion has only one charge (monovalent) and is not rejected by the RO membrane as well as calcium for example, which has two charges.
Likewise, this is why an RO system does not remove gases such as CO2 very well because they are not
highly ionized (charged) while in solution and have a very low molecular weight. Because an RO system does not remove gases, the permeate water can have a slightly lower than normal pH level depending on CO2 levels in the feed water as the CO2 is converted to carbonic acid.
Below is a diagram outlining the process of Reverse Osmosis. When pressure is applied to the concentrated solution, the water molecules are forced through the semi‐permeable membrane and the contaminants are not allowed through.
How does Reverse Osmosis work?
Reverse osmosis works by using a high pressure pump to increase the pressure on the salt side of the RO and force the water across the semi‐permeable RO membrane, leaving almost all (around 95% to 99%) of dissolved salts behind in the reject stream. The amount of pressure required depends on the salt concentration of the feed water. The more concentrated the feed water, the more pressure is required to overcome the osmotic pressure.
What will Reverse Osmosis remove from water?
Reverse Osmosis is capable of removing up to 99%+ of the dissolved salts (ions), particles, colloids, organics, bacteria and pyrogens from the feed water (although an RO system should not be relied upon to remove 100% of bacteria and viruses). An RO membrane rejects contaminants based on their size and charge. Any contaminant that has a molecular weight greater than 200 is likely rejected by a properly running RO system.
Likewise, the greater the ionic charge of the contaminant, the more likely it will be unable to pass through the RO membrane. For example, a sodium ion has only one charge (monovalent) and is not rejected by the RO membrane as well as calcium for example, which has two charges.
Likewise, this is why an RO system does not remove gases such as CO2 very well because they are not
highly ionized (charged) while in solution and have a very low molecular weight. Because an RO system does not remove gases, the permeate water can have a slightly lower than normal pH level depending on CO2 levels in the feed water as the CO2 is converted to carbonic acid.
Reverse Osmosis is very effective in treating brackish, surface and ground water for both large and small flows applications. Some examples of industries that use RO water include pharmaceutical, boiler feed water, food and beverage, metal finishing and semiconductor manufacturing to name a few.